![Image result for plos one]() “Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g., cannabinoid receptors (CB1 and CB2) agonists including tetrahydrocannabinol (THC) and cannabidiol (CBD).
“Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g., cannabinoid receptors (CB1 and CB2) agonists including tetrahydrocannabinol (THC) and cannabidiol (CBD).
The combined use of THC and CBD confers greater benefits, as CBD enhances the effects of THC and reduces its psychotropic activity. We assessed the relationship between the expression levels of CB1 and CB2 to the clinical features of a cohort of patients with NSCLC, and the effect of THC and CBD (individually and in combination) on proliferation, EMT and migration in vitro in A549, H460 and H1792 lung cancer cell lines.
METHODS:
Expression levels of CB1, CB2, EGFR, CDH1, CDH2 and VIM were evaluated by quantitative reverse transcription-polymerase chain reaction. THC and CBD (10-100 μM), individually or in combination (1:1 ratio), were used for in vitro assays. Cell proliferation was determined by BrdU incorporation assay. Morphological changes in the cells were visualized by phase-contrast and fluorescence microscopy. Migration was studied by scratch recolonization induced by 20 ng/ml epidermal growth factor (EGF).
RESULTS:
The tumor samples were classified according to the level of expression of CB1, CB2, or both. Patients with high expression levels of CB1, CB2, and CB1/CB2 showed increased survival reaching significance for CB1 and CB1/CB2 (p = 0.035 and 0.025, respectively).
Both cannabinoid agonists inhibited the proliferation and expression of EGFR in lung cancer cells, and CBD potentiated the effect of THC. THC and CBD alone or in combination restored the epithelial phenotype, as evidenced by increased expression of CDH1 and reduced expression of CDH2 and VIM, as well as by fluorescence analysis of cellular cytoskeleton.
Finally, both cannabinoids reduced the in vitro migration of the three lung cancer cells lines used.
CONCLUSIONS:
The expression levels of CB1 and CB2 have a potential use as markers of survival in patients with NSCLC. THC and CBD inhibited the proliferation and expression of EGFR in the lung cancer cells studied. Finally, the THC/CBD combination restored the epithelial phenotype in vitro.”
https://www.ncbi.nlm.nih.gov/pubmed/32049991
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0228909
 “This study evaluated the potential of combined cannabis constituents to reduce nausea.
“This study evaluated the potential of combined cannabis constituents to reduce nausea. “Cannabis was used for cancer patients as early as about 2500 years ago.
“Cannabis was used for cancer patients as early as about 2500 years ago. “Tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabinol (CBN) affect the human endocannabinoid system.
“Tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabinol (CBN) affect the human endocannabinoid system. “The aim of this review article is to summarize current knowledge about the role of cannabinoids and cannabinoid receptors in tumor disease modulation and to evaluate comprehensively the use of cannabinoids in cancer patients.
“The aim of this review article is to summarize current knowledge about the role of cannabinoids and cannabinoid receptors in tumor disease modulation and to evaluate comprehensively the use of cannabinoids in cancer patients. “Recent changes to the legal status of marijuana in Canada warrant a review of the information that patients and families are accessing online regarding the role of
“Recent changes to the legal status of marijuana in Canada warrant a review of the information that patients and families are accessing online regarding the role of  “In recent years, the endocannabinoid system has received great interest as a potential therapeutic target in numerous pathological conditions.
“In recent years, the endocannabinoid system has received great interest as a potential therapeutic target in numerous pathological conditions.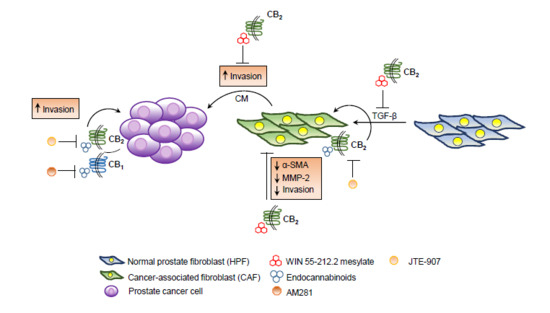
 “In the last decade, we have observed an increased public and scientific interest in the clinical applications of medical cannabis.
“In the last decade, we have observed an increased public and scientific interest in the clinical applications of medical cannabis. “Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol.
“Cannabinoids are a group of structurally heterogeneous but pharmacologically related compounds, including plant-derived cannabinoids, synthetic substances and endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol. “Novel anticancer medicines, including targeted therapies and immune checkpoint inhibitors, have greatly improved the management of cancers. However, both conventional and new anticancer treatments induce cardiac adverse effects, which remain a critical issue in clinic.
“Novel anticancer medicines, including targeted therapies and immune checkpoint inhibitors, have greatly improved the management of cancers. However, both conventional and new anticancer treatments induce cardiac adverse effects, which remain a critical issue in clinic. “Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g.,
“Patients with non-small cell lung cancer (NSCLC) develop resistance to antitumor agents by mechanisms that involve the epithelial-to-mesenchymal transition (EMT). This necessitates the development of new complementary drugs, e.g.,  “Metastatic breast cancer is prevalent worldwide, and one of the most common sites of metastasis are long bones. Of patients with disease, the major symptom is pain, yet current medications fail to adequately result in analgesic efficacy and present major undesirable adverse effects.
“Metastatic breast cancer is prevalent worldwide, and one of the most common sites of metastasis are long bones. Of patients with disease, the major symptom is pain, yet current medications fail to adequately result in analgesic efficacy and present major undesirable adverse effects. “Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).
“Breast cancer (BC) is the most common cancer in women worldwide. Approximately 70-80% of BCs express estrogen receptors (ER), which predict the response to endocrine therapy (ET), and are therefore hormone receptor-positive (HR+).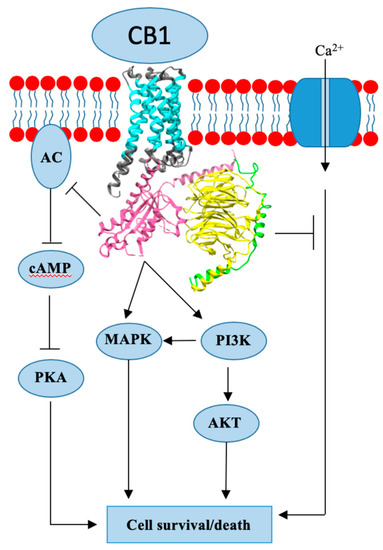

 “The weak but noteworthy presence of (poly)phenols in hemp seeds has been long overshadowed by the essential polyunsaturated fatty acids and digestible proteins, considered responsible for their high nutritional benefits. Instead, lignanamides and their biosynthetic precursors, phenylamides, seem to display interesting and diverse biological activities only partially clarified in the last decades. Herein, negative mode HR-MS/MS techniques were applied to the chemical investigation of a (poly)phenol-rich fraction, obtained from hemp seeds after extraction/fractionation steps. This extract contained phenylpropanoid amides and their random oxidative coupling derivatives, lignanamides, which were the most abundant compounds and showed a high chemical diversity, deeply unraveled through high resolution tandem mass spectrometry (HR-MS/MS) tools.
“The weak but noteworthy presence of (poly)phenols in hemp seeds has been long overshadowed by the essential polyunsaturated fatty acids and digestible proteins, considered responsible for their high nutritional benefits. Instead, lignanamides and their biosynthetic precursors, phenylamides, seem to display interesting and diverse biological activities only partially clarified in the last decades. Herein, negative mode HR-MS/MS techniques were applied to the chemical investigation of a (poly)phenol-rich fraction, obtained from hemp seeds after extraction/fractionation steps. This extract contained phenylpropanoid amides and their random oxidative coupling derivatives, lignanamides, which were the most abundant compounds and showed a high chemical diversity, deeply unraveled through high resolution tandem mass spectrometry (HR-MS/MS) tools. “The global cancer burden is significantly increasing at an alarming rate affecting patients, relatives, communities, and health-care system. Cancer patients require adequate pain relief and palliative care throughout the life course, especially in terminal illness. Although opioid treatment is successful in majority of patients, around 40% do not achieve enough analgesia or are prone to serious side effects and toxicity. The treatment of medical conditions with cannabis and
“The global cancer burden is significantly increasing at an alarming rate affecting patients, relatives, communities, and health-care system. Cancer patients require adequate pain relief and palliative care throughout the life course, especially in terminal illness. Although opioid treatment is successful in majority of patients, around 40% do not achieve enough analgesia or are prone to serious side effects and toxicity. The treatment of medical conditions with cannabis and  “Glioblastoma multiforme (GBM) is the most frequent and aggressive malignant brain tumour, with a poor prognosis despite available surgical and radio-chemotherapy, rising the necessity for searching alternative therapies. Several preclinical studies evaluating the efficacy of
“Glioblastoma multiforme (GBM) is the most frequent and aggressive malignant brain tumour, with a poor prognosis despite available surgical and radio-chemotherapy, rising the necessity for searching alternative therapies. Several preclinical studies evaluating the efficacy of  “Given the infancy and evolving complexity of medicinal marijuana, an evolving political landscape, and the growing frequency of its use in cancer care, it is important for oncologists to be actively engaged in developing and successfully implementing clinical trials focusing on medical marijuana.
“Given the infancy and evolving complexity of medicinal marijuana, an evolving political landscape, and the growing frequency of its use in cancer care, it is important for oncologists to be actively engaged in developing and successfully implementing clinical trials focusing on medical marijuana. “The endocannabinoid system (ECS) comprises the canonical receptor subtypes CB1R and CB2R and endocannabinoids (anandamide, AEA and 2-arachidonoylglycerol, 2-AG), and a “non-canonical” extended signaling network consisting of: (i) other fatty acid derivatives; (ii) the defined “ionotropic cannabinoid receptors” (TRP channels); other GPCRs (GPR55, PPARα); (iii) enzymes involved in the biosynthesis and degradation of endocannabinoids (FAAH and MAGL); and (iv) protein transporters (FABP family).The ECS is currently a hot topic due to its involvement in cancer and pain.
“The endocannabinoid system (ECS) comprises the canonical receptor subtypes CB1R and CB2R and endocannabinoids (anandamide, AEA and 2-arachidonoylglycerol, 2-AG), and a “non-canonical” extended signaling network consisting of: (i) other fatty acid derivatives; (ii) the defined “ionotropic cannabinoid receptors” (TRP channels); other GPCRs (GPR55, PPARα); (iii) enzymes involved in the biosynthesis and degradation of endocannabinoids (FAAH and MAGL); and (iv) protein transporters (FABP family).The ECS is currently a hot topic due to its involvement in cancer and pain.